Our interest in biomaterials ranges from design and development of synthetic analogues of ECM to medical devices to stimuli-responsive systems and actuators. In the case of the former, we not only design biomaterials with tissue-specific functions and properties, but also use these engineered ECM mimetics to unravel the effect of the physicochemical properties of ECM on various cellular functions in health and disease. We also use these engineered materials to deliver cells and promote their in vivo integration and/or activate endogenous tissue repair. Below we provide a brief discussion of various research activities centered around biomaterials and polymers.
Engineered ECM mimetics:

It is now well established that ECM-based cues play a critical role in modulating various cellular functions such as adhesion, differentiation, and functional tissue formation, though the underlying mechanisms remain largely unknown. In an effort to dissect the role of biophysical and biochemical cues of the ECM in directing stem cell commitment, we are developing artificial ECMs recapitulating various physicochemical cues of the native tissue. Some examples include development of hydrogels with varying interfacial properties to regulate adhesion and migration of stem cells, biomaterials for ex vivo expansion of human ESCs and iPSCs, biomaterials to activate endogenous cell-mediated tissue repair, and hydrogel-based actuators for stem cell culture.
H Kang, Y Zeng, and S Varghese. "Functionally graded multilayer scaffolds for in vivo osteochondral tissue engineering." Acta Biomater 78 (2018): 365-377.
H Kang, Y-RV Shih, Y Hwang, C Wen, V Rao, T Seo, and S Varghese. "Mineralized gelatin methacrylate-based matrices induce osteogenic differentiation of human induced pluripotent stem cells." Acta Biomater 10, no. 12 (2014): 4961-4970.
C-W Chang, Y Hwang, D Brafman, T Hagan, C Phung, and S Varghese. "Engineering cell-material interfaces for long-term expansion of human pluripotent stem cells." Biomaterials 34, no. 4 (2013): 912-921.
HL Lim, JC Chuang, T Tran, A Aung, G Arya, and S Varghese. "Dynamic electromechanical hydrogel matrices for stem cell culture." Advanced Functional Materials 21, no. 1 (2011): 55-63.
Biomaterials to unravel molecular mechanism:
Besides directing cell fate, we also utilize the engineered materials as a platformunderstand the molecular and cellular mechanisms through which ECM maintains tissuefunction and homeostasis. For instance, by using dynamic mineralized materials along with stem cells, we have unraveled a previously unknown molecular mechanism, phosphate-ATP-adenosine metabolic signaling axis, by which the calciumphosphate (CaP)-rich mineral environment in bone tissues supports bone homeostasis. These studies have also shed light onto the role of A2B receptor activity on bone formation vs. fat formation. Specifically, A2B activation promotes osteogenic commitment of bone marrow progenitor cells while their inhibition promotes adipogenesis.

Y-RV Shih, M Liu, SK Kwon, M Iida, Y Gong, N Sangaj, and S Varghese. "Dysregulation of ectonucleotidase-mediated extracellular adenosine during postmenopausal bone loss." Science Advances 5, no. 8 (2019): eaax1387.
H Kang, Y-RV Shih, and S Varghese. "Biomineralized matrices dominate soluble cues to direct osteogenic differentiation of human mesenchymal stem cells through adenosine signaling." Biomacromolecules 16, no. 3 (2015): 1050-1061.
V Rao, YRV Shih, H Kang, H Kabra, and S Varghese. "Adenosine signaling mediates osteogenic differentiation of human embryonic stem cells on mineralized matrices." Frontiers in Bioengineering and Biotechnology 3, no. NOV (2015).
Y-RV Shih, Y Hwang, A Phadke, H Kang, NS Hwang, EJ Caro, S Nguyen, M Siu, EA Theodorakis, NC Gianneschi et al. "Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling." Proc Natl Acad Sci U S A 111, no. 3 (2014): 990-995.
Cell therapy:
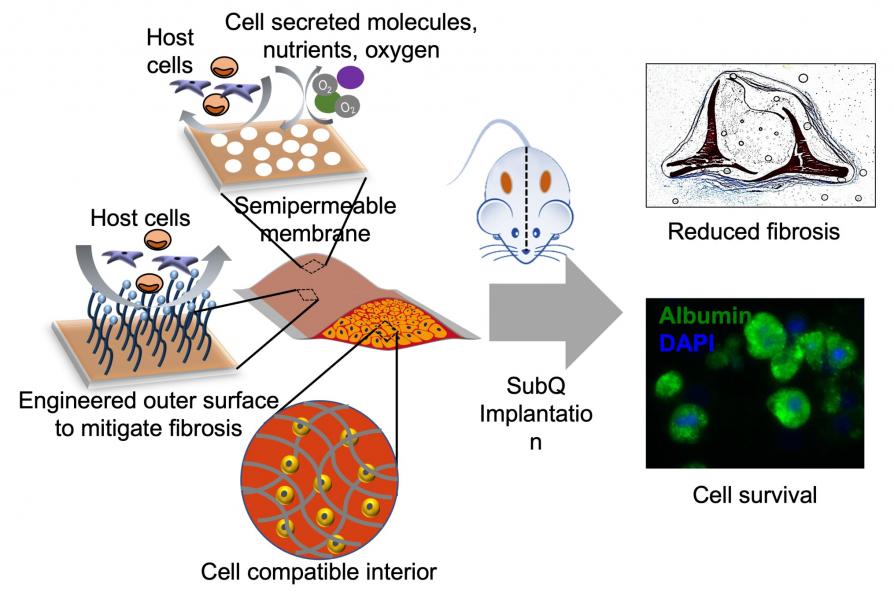
Cell therapy also known as regenerative medicine is considered as a transformative therapy to treat compromised tissues. The goal of cell therapy is to restore or augment the function of compromised organs. Biomaterials have played a key role in advancing cell therapy which includes promoting organ-specific differentiation of stem cells to engraftment and integration of transplanted cells to assist allogenic and xenogeneic cell transplantation. While the differentiation and in vivo engraftment of cells relies on promoting the interaction between the cells and the biomaterial, allogenic and xenogeneic transplantation of cells demands the biomaterial to “hide” the transplanted cells from the host. We have devised biomaterials to promote stem cell differentiation as well as support in vivo survival and function of transplanted cells. A recurring theme in our design is interfacial engineering— i.e., tailoring the cell-matrix interface via molecular engineering of the biomaterial to alter cell attachment and function. One such example is the development of a macroencapsulation device, “cell pouch”, for xenogeneic transplantation of cells in an immunocompetent host.
N Seale, S Ramaswamy, Y-R Shih, I Verma, and S Varghese. "Macroporous Dual-compartment Hydrogels for Minimally Invasive Transplantation of Primary Human Hepatocytes." Transplantation 102, no. 9 (2018): e373-e381.
SK Madhurakka Perikamana, N Seale, J Hoque, JH Ryu, V Kumar, YV Shih, and S Varghese. "Molecularly Tailored Interface for Long-Term Xenogeneic Cell Transplantation." Advanced Functional Materials (2021): 2108221.
Y-R Shih, H Kang, V Rao, Y-J Chiu, SK Kwon, and S Varghese. "In vivo engineering of bone tissues with hematopoietic functions and mixed chimerism." Proceedings of the National Academy of Sciences of the United States of America 114, no. 21 (2017): 5419-5424.
H Kabra, Y Hwang, HL Lim, M Kar, G Arya, and S Varghese. "Biomimetic material-assisted delivery of human embryonic stem cell-derived cells for enhanced in vivo survival and engraftment." ACS Biomaterials Science & Engineering 1 (2015): 7-12.
Localization of pro-regenerative molecules:
In mammals, tissue regeneration is achieved through a cascade of pro-regenerative events. Most organs experience a progressive decline in tissue regenerative ability with aging. The contribution of cell-intrinsic and cell-extrinsic factors on tissue regeneration is clearly established. Rejuvenating the cellular and molecular landscape of the injured tissue environment could be a potential therapeutic approach to activate endogenous tissue repair. Localization of pro-regenerative molecules at the injury site can be achieved by the sequestration of endogenous molecules or by delivering such molecules to the site. In our research, we use both sequestration of endogenous molecules as well as delivery of pro-regenerative molecules to promote healing. For example, we have devised a biomaterial technology to sequester adenosine, a multi-functional osteoanabolic molecule to promote fracture healing. Localization of osteoanabolic adenosine promoted fracture healing. We have also utilized biomaterial (nanocarrier) to deliver adenosine to the bone in order to treat osteoporosis.

J Hoque, Y-RV Shih, Y Zeng, H Newman, N Sangaj, N Arjunji, and S Varghese. "Bone targeting nanocarrier-assisted delivery of adenosine to combat osteoporotic bone loss." Biomaterials 273 (2021).
Y Zeng, Y-RV Shih, GS Baht, and S Varghese. "In Vivo Sequestration of Innate Small Molecules to Promote Bone Healing." Adv Mater 32, no. 8 (2020).
Y Zeng, J Hoque, and S Varghese. "Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair." Acta Biomater 93 (2019): 152-168.
M Kar, Y-R Vernon Shih, DO Velez, P Cabrales, and S Varghese. "Poly(ethylene glycol) hydrogels with cellcleavable groups for autonomous cell delivery." Biomaterials 77 (2016): 186-197.
Self-healing biomaterials and soft robotics:

Another area of our interest is in designing synthetic materials or hydrogels emulating various attributes of biological systems such as sensitivity, self-organization, and self-healing. Our research has resulted in new design principles enabling self-healing in chemically crosslinked hydrogels. We have now extended these approaches to creating self-repairing lubricants and dermal fillers. Our initial studies with the self-repairing HA lubricants showed seamless injectability (due to shear thinning), enhanced lubrication and chondroprotective function. We have recently incorporated some of these design principles and stimuli-responsive materials towardsdevelopment of environment-responsive soft robots.
V Kumar, UH Ko, Y Zhou, J Hoque, G Arya, and S Varghese. "Microengineered Materials with Self‐Healing Features for Soft Robotics." Advanced Intelligent Systems 3, no. 7 (2021): 2100005.iples and stimuli-responsive materials towards the development of environment-responsive soft robots.
A Gilpin, Y Zeng, J Hoque, JH Ryu, Y Yang, S Zauscher, W Eward, and S Varghese. "Self-Healing of Hyaluronic Acid to Improve In Vivo Retention and Function." Adv Healthc Mater (2021).
A Phadke, C Zhang, B Arman, C-C Hsu, RA Mashelkar, AK Lele, MJ Tauber, G Arya, and S Varghese. "Rapid self-healing hydrogels." Proc Natl Acad Sci U S A 109, no. 12 (2012): 4383-4388.
HL Lim, Y Hwang, M Kar, and S Varghese. "Smart hydrogels as functional biomimetic systems." Biomaterials Science 2, no. 5 (2014): 603-618.